LETTER TO THE SHAREHOLDERS
December 3, 2025
Napoleonville, LA IFUS:OTCID
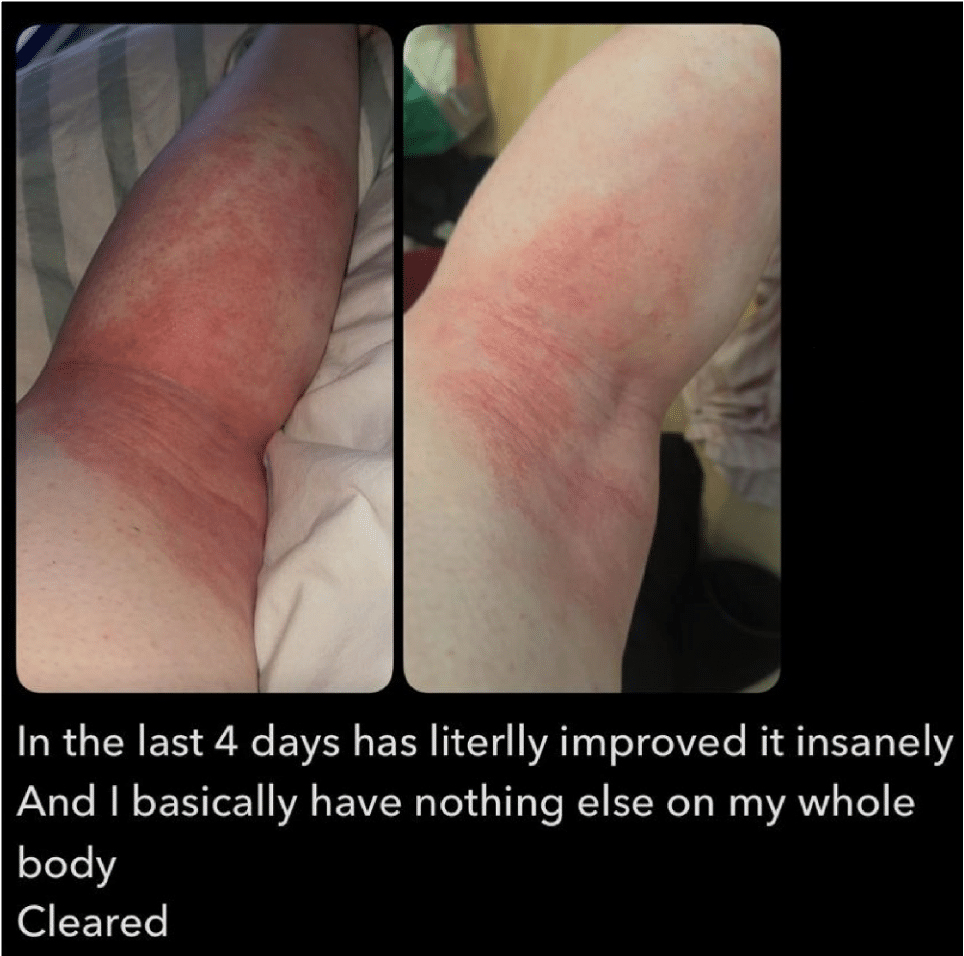
Our customers are offering testimonials as to the efficacy of IFUS Intact Digest™ with Nutri-Mastic™ applied orally and/or topically. Below you will find one such testimony from a customer who applied Intact Digest™ topically to an outbreak of Eczema. However, we want of offer a bit of information prior to the photo.
WHAT CAUSES ECZEMA or ATOPIC DERMATITIS?
Per the Mayo Clinic: “In some people, atopic dermatitis is related to a gene variation that affects the skin’s ability to provide protection. With a weak barrier function, the skin is less able to retain moisture and protect against bacteria, irritants, allergens and environmental factors — such as tobacco smoke.
In other people, atopic dermatitis is caused by too much of the bacteria
Staphylococcus aureus on the skin. This displaces helpful bacteria and disrupts the skin’s barrier function.
A weak skin barrier function might also trigger an immune system response that causes the inflamed skin and other symptoms.
Atopic dermatitis (eczema) is one of several types of dermatitis. Other common types are contact dermatitis and seborrheic dermatitis (dandruff). Dermatitis isn’t contagious.”
Now to the photo provided by a customer who applied Intact Digest™ topically to an Eczema outbreak:
Our IFUS Scientific Team offers additional information about Eczema:
CAN ECZEMA BE CURED?
“There is no known cure for eczema, but it can be managed effectively with various treatments (Ref.1: American Academy of Dermatology Association):
- Topical Treatments: Corticosteroid creams and topical calcineurin inhibitors are commonly used to reduce inflammation and control flare-ups. (Ref. 2: The American Academy of Dermatology Association)
- Moisturizers: Keeping the skin hydrated is essential for managing eczema.
(Ref.1: American Academy of Dermatology Association):
- Immunomodulators: Non-steroidal creams that modify the immune response in the skin are often used when topical steroids are not effective. (Ref.1: American Academy of Dermatology Association):
- Lifestyle Changes: Avoiding triggers, reducing stress, and maintaining a healthy diet can help manage eczema symptoms. (Ref.1: American Academy of Dermatology Association):
- Phototherapy: Exposing the skin to controlled amounts of natural or artificial light can be effective for moderate to severe eczema. (Ref.1: American Academy of Dermatology Association):
- While eczema can be a lifelong condition, with the right treatment and lifestyle changes, many people can manage their symptoms and improve their quality of life. (Ref. 2: The American Academy of Dermatology Association)
Ref. 1: https://my.clevelandclinic.org/health/diseases/9998-eczema
Ref. 2: https://www.aad.org/public/diseases/eczema/childhood/treating/becured
Hence, our IFUS Scientific Team asked the question: Does Chios Mastic Gum affect atopic dermatitis? Here is what the science is suggesting:
“Revealing the Potential of Chios Mastic Gum and Its Constituents for Cosmetic Applications through Chemical Profiling and Biological Evaluation: Chios mastic gum may have potential benefits for atopic dermatitis due to its anti-inflammatory and antibacterial properties, but specific research on its effects for this condition is limited.
Potential Benefits:
Anti-Inflammatory Properties: Chios mastic gum contains compounds that exhibit anti-inflammatory effects, which could theoretically help reduce inflammation associated with atopic dermatitis. Inflammation is a key feature of this skin condition, and reducing it may alleviate symptoms. (Ref. 2)
Antibacterial Effects: The antibacterial properties of mastic gum may help in preventing secondary infections that can occur in atopic dermatitis, where the skin barrier is compromised. This could be beneficial in managing the condition. (Ref. 2)
Some studies suggest that mastic gum can promote skin health and may be used in cosmetic applications to combat issues like acne, which indicates its potential for broader skin-related benefits.” (Ref. 1)
Ref. 1: Stamou, P.; Mikropoulou, E.V.; Chalkiadaki, M.; Basdeki, A.;
Antoniadi, L.; Poigny, S.; Halabalaki, M. Revealing the Potential of Chios Mastic Gum and Its Constituents for Cosmetic Applications through Chemical Profiling and Biological Evaluation. Cosmetics 2024, 11, 155.
https://doi.org/10.3390/cosmetics11050155
Abstract: Chios mastic gum (CMG), the resin of Pistacia lentiscus var. Chia, is a product with great ethnopharmacological and economic significance. This study attempts to investigate, for the first time, the activity of CMG, its fractions and isolated compounds against specific enzymes, which play pivotal roles in the degradation of proteins contained in skin connective tissue. Initially, crude CMG was subjected to extraction, fractionation and isolation through different chromatographic techniques to obtain the acidic and neutral fraction of terpenes. Additionally, the characteristic and major active triterpene acids of CMG, masticadienonic and isomasticadienonic acids (MNA, IMNA) were isolated in pure form. All samples were analysed by means of High-Performance Thin-Layer Chromatography (HPTLC) with four distinct development systems to obtain their constituents’ profile. Finally, samples were tested for their ability to inhibit the elastase and collagenase enzymes. According to our findings, for collagenase, a mixture of MNA and IMNA demonstrated the most potent activity with an IC50 value of 31.07 μg/mL, while for elastase CMG’s acidic fraction provided the most promising results with an IC50 value of 17.30 μg/mL. Overall, these results attempt to fill the gap in scientific knowledge about the use of CMG and its constituents in skincare and cosmetic products.
Ref. 2: Soulaidopoulos S, Tsiogka A, Chrysohoou C, Lazarou E,
Aznaouridis K, Doundoulakis I, Tyrovola D, Tousoulis D, Tsioufis K, Vlachopoulos C, Lazaros G. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients. 2022 Jan 28;14(3):590. doi:
10.3390/nu14030590. PMID: 35276949; PMCID: PMC8838553.
Abstract: Despite the remarkable development of the medical industry in the current era, herbal products with therapeutic potentials arise as attractive alternative treatments. Consequently, Chios mastiha, a natural, aromatic resin obtained from the trunk and brunches of the mastic tree, has recently gained increasing scientific interest due to its multiple beneficial actions.
Chios mastiha is being exclusively produced on the southern part of Chios, a Greek island situated in the northern Aegean Sea, and its therapeutic properties have been known since Greek antiquity. There is now substantial evidence to suggest that mastiha demonstrates a plethora of favorable effects, mainly attributed to the anti-inflammatory and anti-oxidative properties of its components. The main use of mastiha nowadays, however, is for the production of natural chewing gum, although an approval by the European Medicines Agency for mild dyspeptic disorders and for inflammations of the skin has been given. The aim of this article is to summarize the most important data about the therapeutic actions of Chios mastiha and discuss future fields for its medical application.
The work published by S. Soulaidopoulos and team provides insight into the specific phytochemical compounds found in Chios Mastic Gum (See Table 1 below):
Nutrients. 2022 Jan 28;14(3):590. doi: 10.3390/nu14030590
Table 1. Compounds of Chios mastic.
Compound
1,4-poly-β-myrcene
20(S)-3β-acetoxy-20-hydroxydammara-24-ene
3-oxo-28-norlup-20 (29)-ene
3β-hydroxy-28-norolean-12-ene
3-oxo-28-norolean-12-ene
3-oxo-dammara-20 (21),24-diene
(8R)-3-Oxo-8-hydroxy-polypoda13E,17E,21-triene
3-oxo-malabarica-14(26),17E,21-triene
3β-hydroxymalabarica-14(26),17E,21-triene
banillic acid
gallic acid trans-cinnamomic acid isomasticadienonic acid
masticadienolic acid moronic acid
oleanolic acid
oleanolic aldehyde
p-hydroxy-benzoic acid
p-hydroxy-phenylacetic acid
tirucallol
tyrosol
Your IFUS Scientific Team is in the process of exploring the effects of each of these phytochemicals on human, animal, and plant health. Furthermore, the team is exploring the eco-friendly and cost-effective sequestration of Carbon as each of the phytochemicals are produced naturally in the plant and then utilized by humans and animals.
S. Soulaidopoulos and team also provide invaluable data into “Human studies assessing mastic’s effects.” (See Table 2 below):
Nutrients. 2022 Jan 28;14(3):590. doi: 10.3390/nu14030590
Table 2.: Human studies assessing mastic’s effects.
| Study | Design | Effect |
| Kaliora et al. [10] | 10 pts with active CD and 8 healthy • controls 2.2 g of mastic daily for 4 weeks • | -Decrease in CD activity index -decrease in IL-6 and CRP |
| • | -no effect in plasma TNFa | |
| Kaliora et al. [11] | 10 pts with active CD and 8 healthy • controls 2.2 g of mastic daily, 4 weeks | -Reduction of TNF-a secretion by mononuclear cells |
| • | -increase in macrophage migration inhibitory factor | |
| Papada et al. [12,13] | 60 pts with IBD randomized to either 2.8 g • of mastic daily for 3 months or placebo • | -Improvement in IBDQ -Decrease in oxLDL |
| • | -Decrease in plasma cysteine and faecal lysozyme | |
| Papada et al. [14] | 68 pts with IBD randomized to either 2.8 g • of mastic daily for 6 months or placebo | -No impact on serum IL-6, faecal calprotectin and faecal lactoferrin |
| Amerikanou et al. [15] | 129 pts with IBD—68 randomized to •mastic group (2.8 g daily for 6 months for pts in remission and for 3 months for pts in relapse) and 61 to placebo | -Increase in IL-17A |
| Dabos et al. [16] | 148 pts with functional dyspepsia • randomized to either mastic 350 mg tid or placebo for 3 weeks • | -Significant improvement of symptoms-(stomach pain in general, stomach pain when anxious, dull ache in the upper abdomen and heartburn) |
| Kanoni et al. [17] | 98 patients with obesity (BMI ≥ 30 kg/m2) •and NAFLD and randomized to either mastic 2.1 g/day or placebo for 6 months | -Improvement in total antioxidant status of NAFLD pts |
| • | -interaction of mastic with cytokines and antioxidant |
| Study | Design | Effect |
| biomarkers implicated in NAFLD pathogenesis | ||
| Moudi et al. [18] | 147 postpartum women randomized to •topical application of 15 g mastic for 3 days on episiotomy wound or to placebo • | -Higher healing rates of episiotomy wound -no effect on episiotomy pain |
| Triantafyllou et al. [19] | 133 subjects were randomized to either 5 g •mastic powder (high dose) or mastic solution for 18 months | -Decrease in serum total cholesterol, LDL, total lipoprotein (a), apolipoprotein A-1, apolipoprotein B, SGOT, SGPT and γ-GT levels |
| Kartalis et al. [20] | 156 subjects received different doses of •mastic for 8 weeks | -Reduction in TC in subjects receiving crude mastic 1 g/day (highest dose) |
| • | -no effect on LDL, HDL, triglycerides, uric acid and CRP | |
| Kontogiannis et al. [21] | 27 subject (13 hypertensive) randomized to •receive one dose of 2.8 g mastic | -Acute decrease in peripheral and aortic SBP in hypertensive pts |
| • | -no changes in normotensive pts | |
| Kottakis et al. [22] | 5 pts with H. Pylori infection and 3 controls •treated with 1 g of mastic daily for 2 months | -Mastic’s arabinogalactan proteins inhibit neutrophil activation in the presence of H. Pylori neutrophil activating protein |
| Dabos et al. [23] | 52 pts with H. Pylori randomized to receive •either 350 mg tid of mastic for 14 days (Group A), or 1.05 g tid of mastic (Group B) for 14 days, or pantoprazole 20 mg bd plus mastic 350 mg tid for 14 days (Group C) or pantoprazole 20 mg bd plus amoxicillin 1 g bd plus clarithromycin 500 mg bd for 10 days (Group D) | -Eradication of H. pylori was confirmed in 4/13 pts in Group A, in 5/13 in Group B, in 0/13 in Group C and in 10/13 in Group D |
| Study | Design | Effect |
| Bebb et al. [24] | 8 pts with H. Pylori •1 g mastic four times daily for 14 days | -No effect on H. Pylori status |
Pts: patients; CD: Crohn’s disease; IL-6: interleukin-6; CRP: C-reactive protein; TNF-a: tumor necrosis factor-alpha; IBD: inflammatory bowel disease; IBDQ: IBD questionnaire; oxLDL: oxidized LDL; TC: total cholesterol; SBP: systolic blood pressure; tid: three times a day; bd: twice daily; NAFLD: non-alcoholic fatty liver disease.
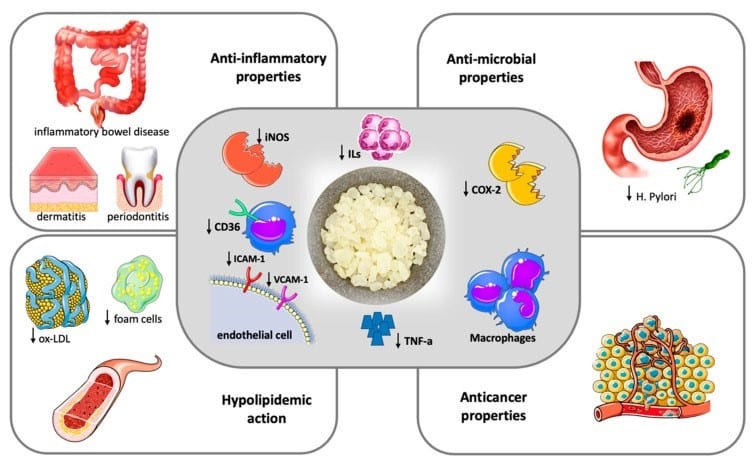
Lastly, like the M. Shabani Team (“The relationship between gut microbiome and human diseases: mechanisms, predisposing factors and potential intervention,” Front Cell Infect Microbiol. 2025 May 6;15:1516010. doi: 10.3389/fcimb.2025.1516010.), the S. Soulaidopoulos and team provide similar information on “Therapeutic potentials of Chios Mastic.”
Nutrients. 2022 Jan 28;14(3):590. doi: 10.3390/nu14030590
Figure 1. Therapeutic potentials of Chios Mastic.
This leads the IFUS Scientific Team to beg the question: Are there specific biochemical pathways that are known to trigger Eczema?
“Various Pathways behind Atopic Eczema and Regulations: The development and progression of atopic eczema are influenced by a complex interplay of genetic, immunological, environmental, and lifestyle factors. Atopic eczema has a strong genetic component, with mutations in the filaggrin gene significantly increasing the risk of developing the condition. These mutations lead to a defective skin barrier, making the skin more sensitive to allergens and immune system reactions. The overactive immune response, particularly through T-helper 2 (Th2) cells, releases cytokines like interleukin-4 (IL-4) and interleukin-13 (IL-13), which contribute to the inflammatory processes observed in eczema. Additionally, the skin microbiome can become imbalanced, with a reduced diversity of beneficial bacteria, contributing to inflammation and exacerbating symptoms. Maintaining skin hydration and using appropriate treatments can help manage atopic eczema by repairing the impaired skin barrier and reducing water loss.” (1 & 2)
Ref. 1: (Pediatrician Specialty Practices:
https://www.federalwaypediatrics.com/blog/eczema-triggers-environmentalvs-internal-causes)
Ref. 2: Sroka-Tomaszewska J, Trzeciak M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int J Mol Sci. 2021 Apr 16;22(8):4130. doi:
10.3390/ijms22084130. PMID: 33923629; PMCID: PMC8074061. (See Figure 2 below):
Int J Mol Sci. 2021 Apr 16;22(8):4130. doi: 10.3390/ijms22084130
Figure 2.
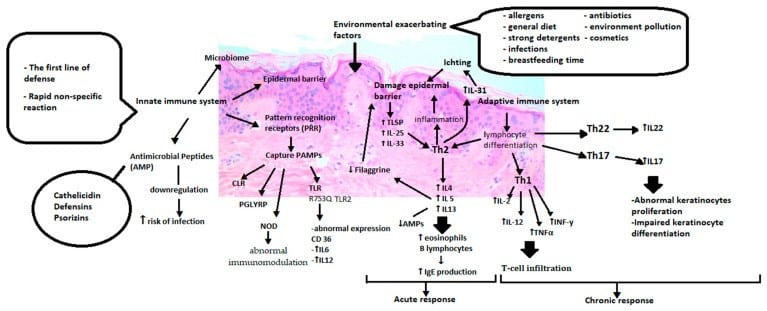
“Immunological aberrations of atopic dermatitis. PRR (Pattern recognition receptors), PAMP (Pathogen-associated molecular patterns), CLR (C lectin receptors), PGLYRP (peptidoglycan recognition proteins), NOD (Nucleotidebinding oligomerization domain), TLR (Toll-like receptors), IL6 (Interleukin 6), IL12 (Interleukin 12),TSLP(Thymic stromal lymphopoietin), IL25
(Interleukin 25), IL33 (Interleukin 33), Th2 (T helper cells 2), IL4 (Interleukin 4), IL5 (Interleukin 5), IL13 (Interleukin 13), IL31 (Interleukin 31), Th1 (T helper cells 1), IL2(Interleukin 2), TNFα (Tumor necrosis factor alpha), INFγ (Interferon gamma), Th22 (T helper cells 22), IL22 (Interleukin 22), Th17 (T helper cells 17), IL17 (Interleukin 17).”
One such biochemical pathway thought to trigger Eczema is the modulation of keratinocyte activation?
“Keratinocyte activation is a significant factor in the pathogenesis of atopic dermatitis, also known as eczema. Activated keratinocytes produce proinflammatory cytokines and chemokines, which play a crucial role in the inflammatory response and recruitment of immune cells to the skin. This interplay between keratinocytes and immune cells is critical for the development and resolution of inflammatory skin diseases, including eczema. In atopic dermatitis, keratinocytes exhibit impaired barrier function, leading to increased permeability and susceptibility to allergens and irritants. Targeting keratinocytes and their interactions with immune cells holds promise as a therapeutic strategy for inflammatory skin diseases, including eczema.”
Ref. 1: Das P, Mounika P, Yellurkar ML, Prasanna VS, Sarkar S,
Velayutham R, Arumugam S. Keratinocytes: An Enigmatic Factor in Atopic Dermatitis. Cells. 2022 May 19;11(10):1683. doi: 10.3390/cells11101683. PMID: 35626720; PMCID: PMC9139464.
Ref. 2: Girolomoni, G., Mascia, F., Dattilo, C., Giannetti, A., Pastore, S. (2006). Keratinocytes in Atopic Eczema. In: Ring, J., Przybilla, B., Ruzicka, T. (eds) Handbook of Atopic Eczema. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/3-540-29856-8_33
This begs one last question in this line of inquiry, that being: Does Chios Mastic Gum have an effect on the modulation of keratinocyte activation?
“Chios Mastic Gum (CGM) has been shown to have a protective effect against oxidative stress and to enhance autophagy in human keratinocytes. This suggests that CGM may have an effect on keratinocyte activation by promoting cell survival and preventing apoptosis under stressful conditions. The study indicates that CGM can help cells to survive by preventing apoptosis and enhancing autophagy, which are crucial for maintaining healthy keratinocyte function.” (1, 2, 3)
Ref. 1: Risako Kishimoto, et.al. “Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis,” Phytomedicine, Volume 91, October 2021, 153679,
https://www.sciencedirect.com/science/article/abs/pii/S0944711321002221
Results: Our findings indicated that topical treatment with mastic significantly ameliorated ear swelling, itch behaviour, immunocyte infiltration, and cytokine production. Histological evaluation confirmed the occurrence of anti-inflammatory responses. The anti-inflammatory and antipruritic effects of topical treatment with mastic (3% and 5%) were further confirmed in a mouse model of atopic dermatitis which was generated by topical application of TDI in NC/Nga mice. Thickness of the back skin, AD score, transepidermal water loss (TEWL), and itch behaviour were measured weekly, and immunocyte differentiation, cytokine determination, and histological changes were also analysed. Mastic treatment significantly attenuated the skin thickness, AD score, TEWL, and itch behaviour. Corroborated reduction was observed in the numbers of T cells and IgE-B cells, as well as in pro-inflammatory cytokine production. The reproducibility of the effects of mastic was confirmed with 1% mastic ointment in a setting similar to the AD mouse model. In vitro evaluation of keratinocytes indicated that mastic pre-exposure induced a significant dosedependent decrease in cytokine production.
Ref. 2: Das P, Mounika P, Yellurkar ML, Prasanna VS, Sarkar S,
Velayutham R, Arumugam S. Keratinocytes: An Enigmatic Factor in Atopic Dermatitis. Cells. 2022 May 19;11(10):1683. doi: 10.3390/cells11101683. PMID: 35626720; PMCID: PMC9139464.
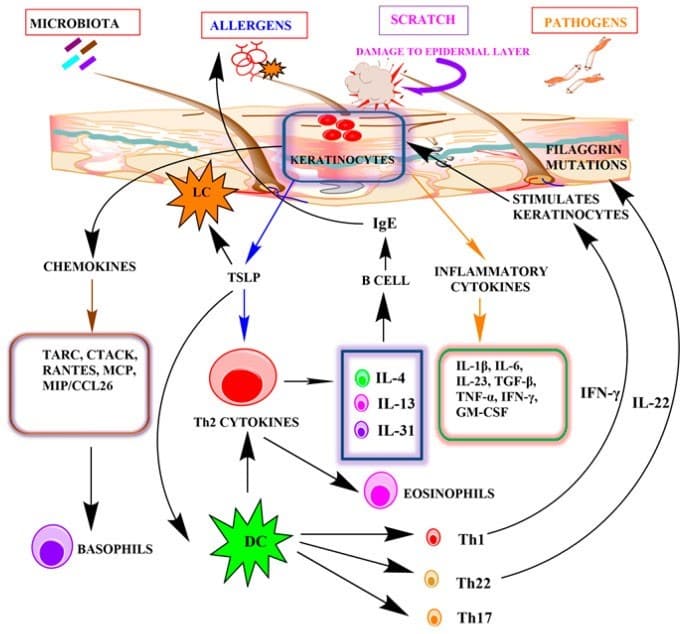
“Conclusions and Future Perspectives: This article illustrates the role of keratinocytes in AD both as a benevolent barrier safeguarding against the pathogenic triggers and as a hostile contributor inducing allergic reactions. Impairment of the innate immune system and epidermal barrier function along with a rise in cutaneous inflammation serve as the major key factors behind the pathogenesis of AD. This emphasizes the role of keratinocytes, the innate immune cells of our skin, in AD. The prevalent treatments are directed towards suppression of the inflammatory reaction and consolidation of the barrier defense. A few targeted approaches, as mentioned in the article, point out the therapeutic importance of keratinocytes. Further insight into certain pathways activated by keratinocytes and the consequent cytokines released would facilitate the development of targeted therapy. However, the complexity of the symptoms and biological processes involved has hindered the process so far.” See Figure 1 and Figure 2 below:
Cells. 2022 May 19;11(10):1683. doi: 10.3390/cells11101683
Figure 1.: Keratinocytes as a part of skin immune defense.

. 2022 May 19;11(10):1683. doi: 10.3390/cells11101683
Figure 2. The role of keratinocytes in the pathogenesis of atopic dermatitis.
The studies provided in this update are again but a snapshot of the science to be reviewed and considered. However, one could reasonably conclude that Chios Mastic Gum contained within Nutri-Mastic™ provides plausible relief for those suffering from Eczema. Furthermore, the critical importance of the ionic mineral formulation within Nutri-Mastic™ should not be discounted, nor should the overall emulsification of these ingredients with one another. This indeed makes NutriMastic™ a proprietary formulation and technology, one that has not been found in any of the research performed to date.
“Seeing pictures of human suffering like that provided in this update candidly is sobering. However, to hear that the products and technologies provided by your company are demonstrating efficacy in providing relief to those suffering remains most rewarding and motivating. Once again, our IFUS Internal and External Scientific Teams are offering plausible explanations as to the efficacy of yet another IFUS Product Line. Our ability to use this information to create sales that will create profitable outcomes for your company is encouraging. We still have much work to do, but are relentless in getting the job done for you and for all of our stakeholders,” said Marc Walther, CEO of Impact Fusion International.
Once more, we are “Back to work!”
The information provided is for informational purposes only and is not intended as medical advice. Our products are not intended to diagnose, treat, cure, or prevent any medical condition. Always consult with a qualified healthcare professional before starting any new supplement, diet, or health regimen.
For our customers of both Intact Digest™ and Intact Endurance™ you may now send your testimonials to:
mwalther@impactfusionintl.com We can also be reached at 1-800-775-4130 seven days a week.
About Impact Fusion Internaonal Inc.
Impact Fusion Internaonal, Inc. is in the business of markeng products in the “Health and Wellness” sector of all internaonal markets. It is the company’s mission to invent, develop and market these proprietary products worldwide for the health and well-being of humans and animals.
The informaon contained in this release includes some statement that are not purely historical and that are “forward-looking statements.” Such forward-looking statements include, but are not limited to, statements regarding our and their management’s expectaons, hopes, beliefs, intenons or strategies regarding the future, including our financial condion, results of operaons. In addion, any statements that refer to projecons, forecasts or other characterizaons of future events or circumstances, including any underlying assumpons, are forward-looking statements. The words “ancipates,” “believes,”
“connue,” “could,” “esmates,” “expects,” “intends,” “may,” “might,” “plans,” “possible,” “potenal,” “predicts,” “projects,” “seeks,” “should,” “would” and similar expressions, or the negaves of such terms, may idenfy forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. The forward-looking statements contained in this release are based on current expectaons and beliefs concerning future developments and the potenal effects on the pares and the corporate and administrave transacons. Forward-looking statements involve known and unknown risks, uncertaines and other factors, which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements and represent our management’s beliefs and assumpons only as of the date hereof. Except as required by law, we assume no obligaon to update these forward-looking statements, even if new informaon becomes available in the future.
Contact:
Impact Fusion Internaonal Inc.
204 Highway 1011
Napoleonville LA 70390
1-800-775-4130
Email: mwalther@impacusionintl.comhtps://www.impacusionbrands.com/brands
Updates can be found at the official Impact Fusion Twiter account @impacusionI
#Foodintelligence #NewMexico #healthiercatle #Screwworms
#Intact #Digeson #Endurance #Germany #Colorado
#legislaon #bagasse #drought #SUAREC #Louisiana
#greenhousegases #methanegas #catle #dairy #Texasfloods
#Texaswildfires $Waygu #India #Black Farmers Naonal
Associaon #Supreme AG™ #SGP+™ #Oklahoma
#KECO 96.5 FM radio #India #Australia #Brazil #Argenna #Canada #Vietnam

 My Account
My Account
You must be logged in to post a comment.